Phathom, (PHAT), down on regulatory update on Vonoprazan NDA
Phathom Pharmaceuticals, Inc. PHAT reported that the FDA had notified the company it would not take action on Phathom’s new drug application (NDA), for vonoprazan, on or before the target date. In response to this news, shares fell 31.11% on January 4.
The NDA is currently being reviewed to approve the candidate for treatment of erosive gastrocitis. Jan 11, 2023 is the current target date.
Vonoprazan is an oral small molecule potassium-competitive acid blocker, or P-CAB. P-CABs are an entirely new class of medicines that inhibit stomach acid production.
Phathom Pharmaceuticals, Inc. Prices and Consensus
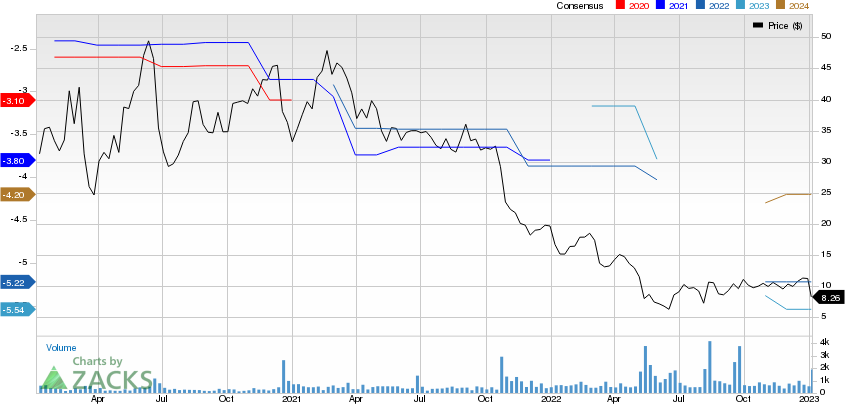
Phathom Pharmaceuticals, Inc. price-consensus-chart | Phathom Pharmaceuticals, Inc. Quote
Phantom reported in August that it had found trace amounts of N-nitrosovonoprazan (NVP) in commercial batches. It was working closely to get approval from the FDA for a proposed acceptable daily consumption limit, test method and controls to remove this impurity before releasing vonoprazan products to the marketplace. NVP is allowed to be consumed daily by FDA at 96ng/day. The FDA requested stability data to prove that NVP levels remain below this limit for the entire shelf-life of the product.
Therefore, Phathom is currently working with FDA to generate additional stability data. Phathom has decided to stop expecting product launches for H. pilori and erosive stomachitis in the first three months of 2023.
FDA approved vonoprazan triple therapy under the brand Voquezna Triple PAK on May 3, 2022. Voquezna dual therapy under the brand Voquezna DUAL PAK was also approved for treatment of H. Pylori infection in adults under the brand Voquezna DUAL PAK on May 3.
Shares have plunged 52.4% in the year so far compared with the industry’s decline of 16.6%.
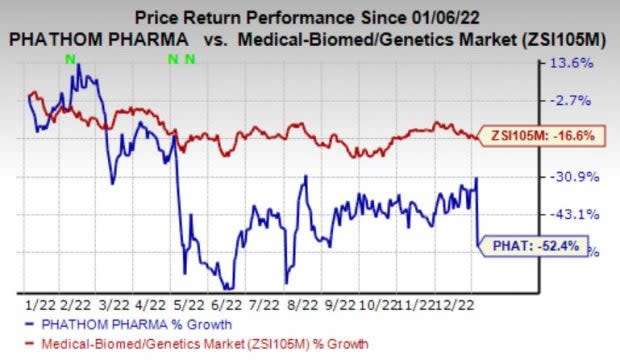
Image Source: Zacks Investment Research
The company announced that it has completed enrollment in the PHALCON NERD daily dosing Phase III study of vonoprazan for non-erosive gastroesophageal acid reflux disease (NERD) last October. In total, 776 patients suffering from NERD were enrolled in the trial at various sites throughout the United States. The current quarter will provide data on the primary endpoint, and full results of the trial will be available later in the year.
The potential success of the study will be the basis for a supplemental drug application (sNDA), for vonoprazan once-daily for the treatment of symptomatic NERD among adults. Submission is planned for 2023.
Takeda Pharmaceutical Company Limited TAK has developed vonoprazan. It has been approved for marketing in many countries in Asia, Latin America and Russia. Phathom licensed the rights to vonoprazan from Takeda in the United States, Europe and Canada in May 2019.
Zacks Rank and Stocks To Consider
Phathom currently holds a Zacks #2 (Buy) rank. Other top-ranked stocks within the biotech sector include Syndax Pharmaceuticals, Inc. SNDX Immunocore Holdings plc IMCR. Both have a Zacks rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
The loss per share estimates of Syndax Pharmaceuticals in 2022 and 2023 for the current 60 days have declined by 11 cents each and 20 cents respectively. Syndax Pharmaceuticals’ earnings exceeded estimates in three quarters, and they met them on the other occasion. SNDX saw an average earnings surprise of 95.39%.
In the last 60 days, loss per share estimates for Immunocore shaved by 77 and 92 cents. The earnings of Immunocore exceeded estimates in three quarters, and they missed the rest. IMCR had an average earnings surprise rate of 68.34%.
Get the most recent Zacks Investment Research recommendations. You can now download 7 Best Stocks to Watch in the Next 30 days. Click to get this free report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report
Phathom Pharmaceuticals, Inc. (PHAT) : Free Stock Analysis Report
Immunocore Holdings PLC Sponsored ADR (IMCR) : Free Stock Analysis Report